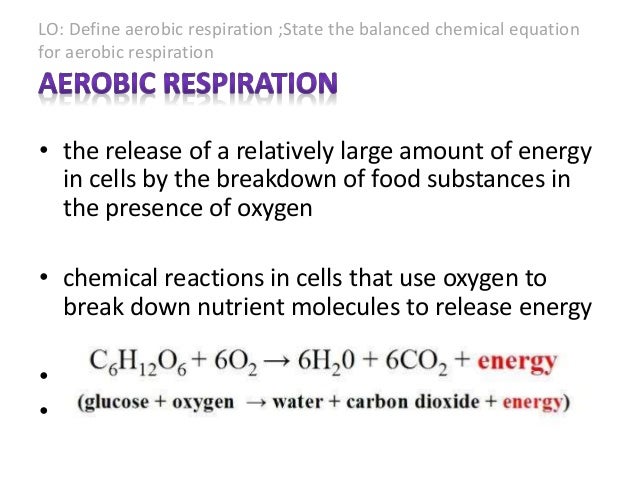
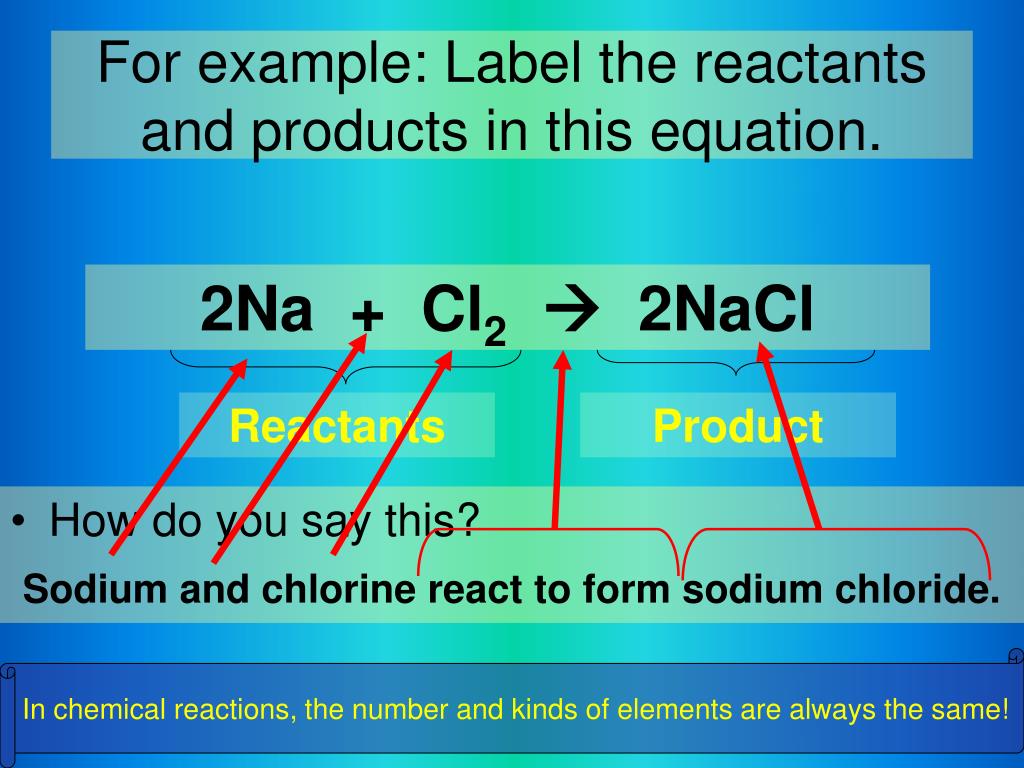
41 chemical equation with labels
Chemical Equation | Reactants And Products In Chemical Reactions We will first take the maximum number of atoms present on either side of the reaction, that we can find on product side. (Oxygen = 4). Then we multiply the number of oxygen atoms on reactant side by 4, such that the number of oxygen atoms on both sides of the reaction is balanced. The equation becomes F e + 4 H 2 O → F e 3 O 4 + H 2 Arrows chemical equations - TeX - LaTeX Stack Exchange I want to write the following equation; note the arrow underneath BaSO_4 and the H_2O over the \\longrightarrow. Is that possible with the chemist package, which I already use for my chemical equat...
Align and label in chemical equation - LaTeX Stack Exchange For the first objective, change from an align* to an align environment and use \notag directives on lines 1, 4, and 5.
Chemical equation with labels
Balancing Different Types of Chemical Equations - Quizlet HINT: Element Fe = 1 atom in reactants, 3 atoms in products. Element H = 2 atoms in reactants, 2 atoms in products. Element O = 1 atom in reactants, 4 atoms in products. The elements iron and oxygen in the chemical equation are not balanced. Add appropriate coefficients until the numbers and kinds of atoms on both sides of the equation are the ... Factor-Label Method in Chemistry: Definition, Examples & Practice ... The conversion factor in this case is expressed as 1 GB = 1 * 10^9 bytes (note that 1 * 10^9 is an example of scientific notation, a way of writing large numbers that expresses them as powers of... Chemical Equations Flashcards | Quizlet Chemical Reaction. process in which bonds between atoms are broken and new bonds are formed. Coefficient. indicates the # of each type of molecule (# that multiplies a term in an equation). ex. 6H2O means there are six water molecules. Subscript. the small 2 (subscript) in H2O is the # of hydrogen atoms. Reactant.
Chemical equation with labels. How to Number or Label Equations in Microsoft Word Open your document and select your first equation. On the References tab, click "Insert Caption" from the Captions section of the ribbon. In the Caption pop-up window, select "Equation" next to Label. This sets both the word and the number as the caption. Optionally, select a Position for the caption and click "OK" to apply the caption. chemical equation | Yeah Chemistry Oct 06, 2011 · Formulas of the reactants appear on the left side of the equation; those of products are written on the right. In many cases it is useful to indicate the state or phases of the substance in an equation.You use the following phase labels: (g) = gas , (l) = liquid , (s) = solid , (aq) = water solution Chapter 4 Quantities of reactants and products Phase labels: letters written in parenthesis after a reactant or product to indicate whether the substance is a solid (s), liquid (l), gas (g) or dissolved in ... what it chemical equation?? 1. Label your initials on | Chegg.com Question: what it chemical equation?? 1. Label your initials on the top portion of your larger test tube (25x150 mm), and place the tube into a 150 mL beaker. Tare the beaker/tube, then use a spoon to carefully add approximately 0.6 g Maleic acid into the bottom of the test tube. Record the exact mass and appearance. 2.
A write a chemical equation for the dissolution of 18. A science teacher needs to determine the concentration of an old, unmarked bottle of hydrochloric acid. She prepares a base titrant by dissolving 40.0 g of potassium hydroxide in 500.0 mL of water. The base is pipetted into a 28.00 mL sample of the acid, which also contains phenolphthalein as an indicator. The solution turns from clear to pink after the addition of 7.34 mL of base. Label the parts of the chemical equation with the appropriate ... Label the parts of the chemical equation with the appropriate descriptions. Sr(NO3)2(aq) + K2CO3(aq) ⟶ SrCO3(s) + 2KNO3(aq). answer bank-. 4.1 Writing and Balancing Chemical Equations - Chemistry Begin by identifying formulas for the reactants and products and arranging them properly in chemical equation form: CO2(aq)+NaOH(aq) Na2CO3(aq)+H2O(l) (unbalanced) CO 2 ( a q) + NaOH ( a q) Na 2 CO 3 ( a q) + H 2 O ( l) ( unbalanced) Label the chemical Equation worksheet - Liveworksheets.com Label the chemical Equation Label the parts of the chemical Equation ID: 1570824 Language: English School subject: Science Grade/level: 5 Age: 7-9 Main content: Chemical Equation Other contents: NA Add to my workbooks (0) Download file pdf Embed in my website or blog
Solved write a balanced chemical equation including phase | Chegg.com See the answer write a balanced chemical equation including phase labels for the reaction between aqueous copper (II) nitrate and aqueous sodium hydroxide Best Answer 100% (1 rating) 1] write equation in words first, you have all the products given except there is also water in the first. 2] rewrite, subs … View the full answer How do you Write a Chemical Equation? - A Plus Topper Step 2: Thus, the word equation is Magnesium + Oxygen Magnesium oxide Step 3: Replacing the names with symbols and formulae, we get the chemical equation as Mg + O 2 MgO Step 4: The number of atoms of the elements are To balance oxygen on both sides, multiply RHS by 2, i.e., Mg + O 2 2MgO Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... This is an example of a chemical equation, which is a concise way of representing a chemical reaction. ... phase label. To balance this equation, we need two phosphate ions and three calcium ions; we end up with six water molecules to balance the equation: 2H 3 PO 4 (aq) + 3Ca(OH) 2 (aq) → 6H 2 O(ℓ) + Ca 3 (PO 4) 2 (s) This chemical ... What are the Parts of a Chemical Equation? - Life Persona The steps followed to write a chemical equation are: - Reagents and reaction products are identified and noted. - The formula or symbols of the reagents are written on the left side with a '+' sign between them. - The formula (s) of the products are written on the right side with a '+' sign between them.
Examples of Balanced Chemical Equations - ThoughtCo 2 AgI + Na 2 S → Ag 2 S + 2 NaI 2 silver iodide + 1 sodium sulfide yields 1 silver sulfide + 2 sodium iodide Ba 3 N 2 + 6 H 2 O → 3 Ba (OH) 2 + 2 NH 3 3 CaCl 2 + 2 Na 3 PO 4 → Ca 3 (PO 4) 2 + 6 NaCl 4 FeS + 7 O 2 → 2 Fe 2 O 3 + 4 SO 2 PCl 5 + 4 H 2 O → H 3 PO 4 + 5 HCl 2 As + 6 NaOH → 2 Na 3 AsO 3 + 3 H 2
What are Chemical Equations? Detailed Explanation, Examples A few examples of chemical equations are listed in bulleted text below. PCl5 + 4H2O → H3PO4 + 5HCl SnO2 + 2H2 → 2H2O + Sn TiCl4 + 2H2O → TiO2 + 4HCl H3PO4 + 3KOH → K3PO4 + 3H2O Na2S + 2AgI → 2NaI + Ag2S How do you write chemical formulas? There’s a particular way of writing what’s in a molecule called a chemical formula.
What Are Chemical Equations? - ThoughtCo This can be seen in the following equation: 2 H 2 (g) + O 2 (g) → 2 H 2 O (l) Hydrogen and oxygen are indicated by (g), which means they are gases. Water is marked (l), which means it is a liquid. Another symbol you may see is (aq), which means the chemical species is in water — or an aqueous solution.
Chapter 4: Chemical Reactions and Equations In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as ...
3.1: Chemical Equations - Chemistry LibreTexts 15 Jun 2022 — A chemical reaction is described by a chemical equation, an expression that gives the identities and quantities of the substances involved in a ...
Chemical Equation Balancer The equation will now look like this: 4Na + O 2 = 2Na 2 O This equation is a balanced equation because there is an equal number of atoms of each element on the left and right hand sides of the equation. Example #2 (Complex) P 4 + O 2 = 2P 2 O 5 This equation is not balanced because there is an unequal amount of O's on both sides of the equation.
Chemical Formula Labels Teaching Resources Results 1 - 24 of 87 — In this worksheet, students will identify the parts of a chemical formula (coefficients, subscripts) and label the parts of a chemical ...
Chemical formula - Wikipedia Aluminium sulfate has the chemical formula Al 2 (SO 4) 3. The form of aluminium sulfate hexadecahydrate is Al2(SO4)3·16 H 2 O. Structural formula for butane. Examples of other chemical formulae for butane are the empirical formula C 2 H 5, the molecular formula C 4 H 10 and the condensed (or semi-structural) formula CH 3 CH 2 CH 2 CH 3.
How to Write a Chemical Equation (with Pictures) - wikiHow In a basic double replacement equation you will have 2 cations and 2 anions. The general equation takes the form of AB + CD → AD + CB, where A and C are cations and B and D are anions. You also want to determine the charges of each ion. [11] For example: AgNO 3 + NaCl → ? The cations are Ag +1 and Na+1. The anions are NO31- and Cl1-. 2



Post a Comment for "41 chemical equation with labels"