42 a product featuring a qualified health claim on its label:
Chapter 1 Nutrition Flashcards | Quizlet A product featuring a qualified health claim on its label: a. must have the highest degree of scientific support for that claim. b. must provide an insert with a detailed explanation of the scientific support backing the claim. c. cannot be sold. d. must bear a statement explaining the degree of scientific evidence backing the claim. Prohibiting Qualified Health Claims for Foods - 1library.net Prohibiting Qualified Health Claims for Foods. The Problem. The second way companies have sought to avoid statutory requirements that health claims for foods be based on "significant scientific agreement" is to persuade the FDA to authorize health claims based on any amount of scientific evidence, so long as the claim is accompanied by a disclaimer that the evidence is uncertain.
Experimental Study of Qualified Health Claims: Protocol Protocol for experimental study to evaluate effectiveness of qualified health claim schemes for conveying information to consumers. Experimental Study of Qualified Health Claims: Consumer Inferences about Monounsaturated Fatty Acids from Olive Oil, EPA and DHA Omega-3 Fatty Acids, and Green Tea: Protocol | FDA

A product featuring a qualified health claim on its label:
Qualified Health Claims | FDA For a QHC petition with credible scientific evidence, the FDA issues a Letter of Enforcement Discretion including specific claim language that reflects the level of supporting scientific evidence... EOF Consumer Health Information for Better Nutrition: Attachment A Label Claims for Food & Dietary Supplements; Standards of Identity for Food; Menu and Vending Machine Labeling ; Gluten-Free Labeling of Foods; Changes to the Nutrition Facts Label; Nutrition Labeling Information for Restaurants & Retail Establishments
A product featuring a qualified health claim on its label:. Qualified Health Claims on Package Labels | Semantic Scholar Qualified health claims arising from the landmark 1999 Pearson v Shalala ruling are revealed to be less common than their consumer-equivalent structure- function claims and are significantly overshadowed by structure-function claims for which little scientific evidence exists. Qualified health claims arising from the landmark 1999 Pearson v. Shalala ruling were predicted to be in ... A Product Featuring a Qualified Health Claim on Its Label In other words a health claim can be a label on a product that says how the food is beneficial in helping to prevent or treat some kind of health condition. In fact FDA has considered over 100 structure-function claim notifications regarding NAC and at least one qualified health claim petition for a dietary supplement containing NAC and has not. [Solved] A Product Featuring a Qualified Health Claim on Its Label [Solved] A product featuring a qualified health claim on its label: A)must have the highest degree of scientific support for that claim. B)must provide an insert with a detailed explanation of the scientific support backing the claim. C)cannot be sold. D)must bear a statement explaining the degree of scientific evidence backing the claim. FDA system would allow qualified health claims on labels WASHINGTON (AP -- The government is loosening restrictions on how much scientific proof is required to advertise a food's possible health benefits on its package, a move welcomed by food makers bu…
A product featuring a qualified health claim on its label: a. must bear ... A product featuring a qualified health claim on its label: a. must bear a statement explaining the degree of scientific evidence backing the claim. b. cannot be sold. c. must have the highest degree of scientific support for that claim. d. must provide an insert with a detailed explanation of the scientific support backing the claim. Learn About Qualified Health Claims | Chegg.com A qualified health claim includes a piece of scientific evidence supporting the established relationship between the substance (food or its derived products) and its effects on the health and is authorized by Food and Drug Administration (FDA). It is then approved for use on food labels and must adhere to local language guidelines. Health Claims On Food Labels (Just Now) Health claims on food labels Mil Med. 1994 Mar;159(3):213-7. Author L Tollefson 1 Affiliation 1 Food and Drug Administration, Center for Food Safety and Applied Nutrition, Washington, DC 20204. PMID: 8041466 Abstract Food and drug law requires that the ingredients in most foods be disclosed on their labels, but until recently there ... FCS Chapter 1 Flashcards | Quizlet A product featuring a qualified health claim on its label: a. must have the highest degree of scientific support for that claim. b. must provide an insert with a detailed explanation of the scientific support backing the claim. c. cannot be sold d. must bear a statement explaining the degree of scientific evidence backing the claim.
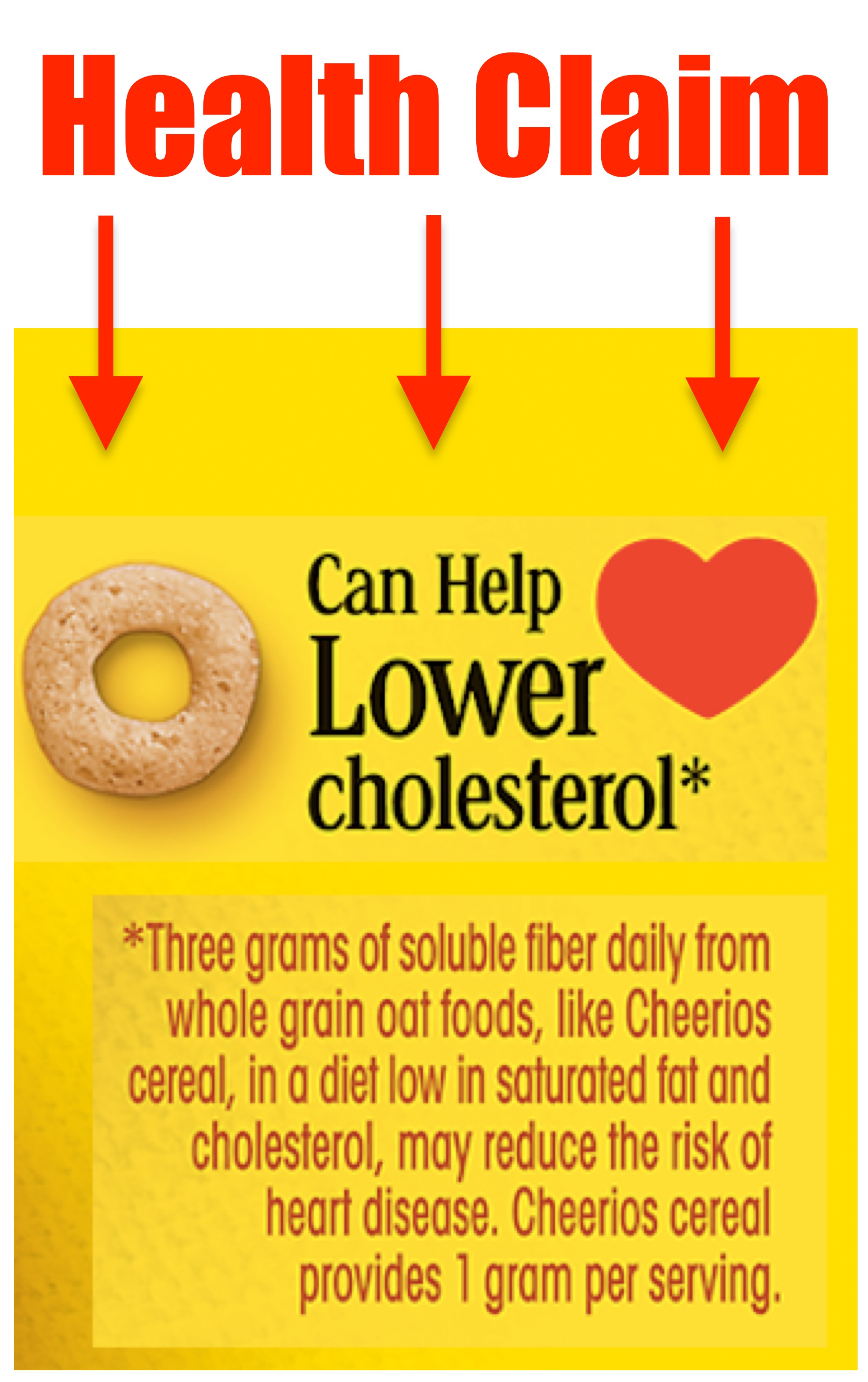
A Product Featuring a Qualified Health Claim on Its Label Product / wallpaper. A Product Featuring a Qualified Health Claim on Its Label By fu_Aileen244 16 Apr, 2022 Post a Comment The Basics Of Food Product Health Claims Labelcalc ... No comments for "A Product Featuring a Qualified Health Claim on Its Label" Post a Comment. Popular Logo Real Madrid Dream League Soccer 2019 Kuchalana ... Live Your Way to Healthy: "Approved" and "Qualified" Claims "Authorized" health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products or dietary supplements to show that a food or food component may reduce the risk of a disease or a health-related condition. An example of an Authorized Health Claim can be found on every box of Cheerios cereal. Questions and Answers on Health Claims in Food Labeling These guidance documents describe a process and procedure for the FDA to systematically evaluate and rank the scientific evidence relevant to a substance/disease relationship that is the subject of... PDF Qualified Health Claims on Package Labels - jstor.org and unreliable health claims." Both predicted extensive use of qualified health claims, but with very different consumer outcomes. Qualified health claims were made possible because of manufacturer activism?either in the court system, as with Pearson and several other cases (e.g., Pearson v. Thompson 2001; Whitaker v. Thompson 2003), or through ...
FDA Takes Steps to Allow Qualified Health Claims on Labels By Keller and Heckman LLP's Packaging Practice Group The Food and Drug Administration announced March 31, 2004, that a qualified health claim will soon appear on product labels for walnuts and the reduced risk of coronary heart disease. "This qualified health claim is part of the FDA's initiative to provide Americans with better information to help them make healthier dietary choices," the ...
Are health claims allowed on food labels? - Sage-Answer Health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products to show that a food or food component may reduce the risk of a disease or a health-related condition. cannot be claims about the diagnosis, cure, mitigation, or treatment of disease; and.
Consumer Health Information for Better Nutrition: Attachment A Label Claims for Food & Dietary Supplements; Standards of Identity for Food; Menu and Vending Machine Labeling ; Gluten-Free Labeling of Foods; Changes to the Nutrition Facts Label; Nutrition Labeling Information for Restaurants & Retail Establishments
EOF
Qualified Health Claims | FDA For a QHC petition with credible scientific evidence, the FDA issues a Letter of Enforcement Discretion including specific claim language that reflects the level of supporting scientific evidence...

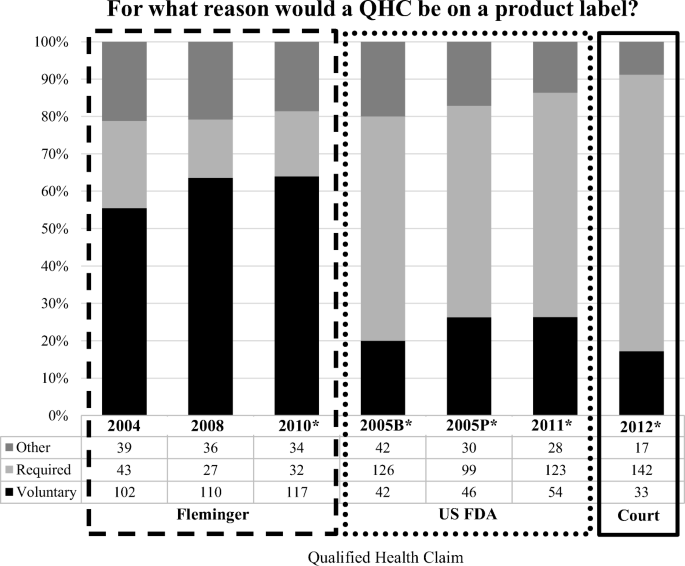

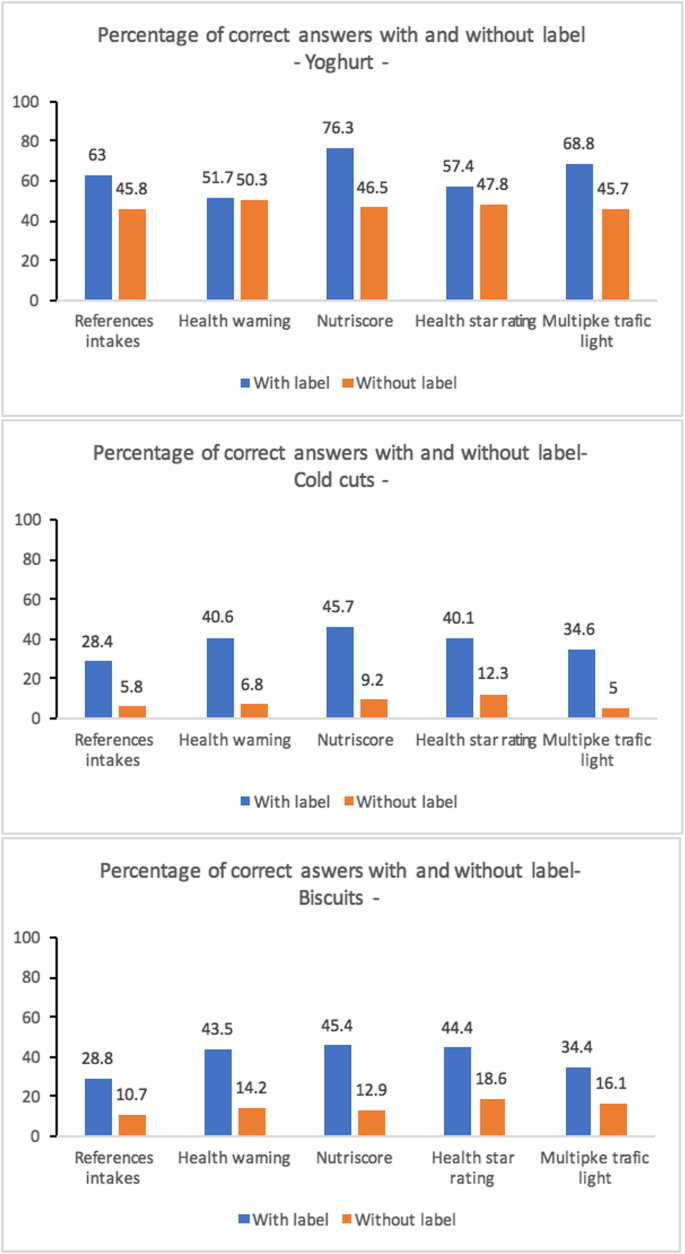
![PDF] The Need for a Legal Distinction of Nutraceuticals ...](https://d3i71xaburhd42.cloudfront.net/747f2ce5a6ea3790d0c05f149a18d2498466eacb/4-Table3-1.png)



















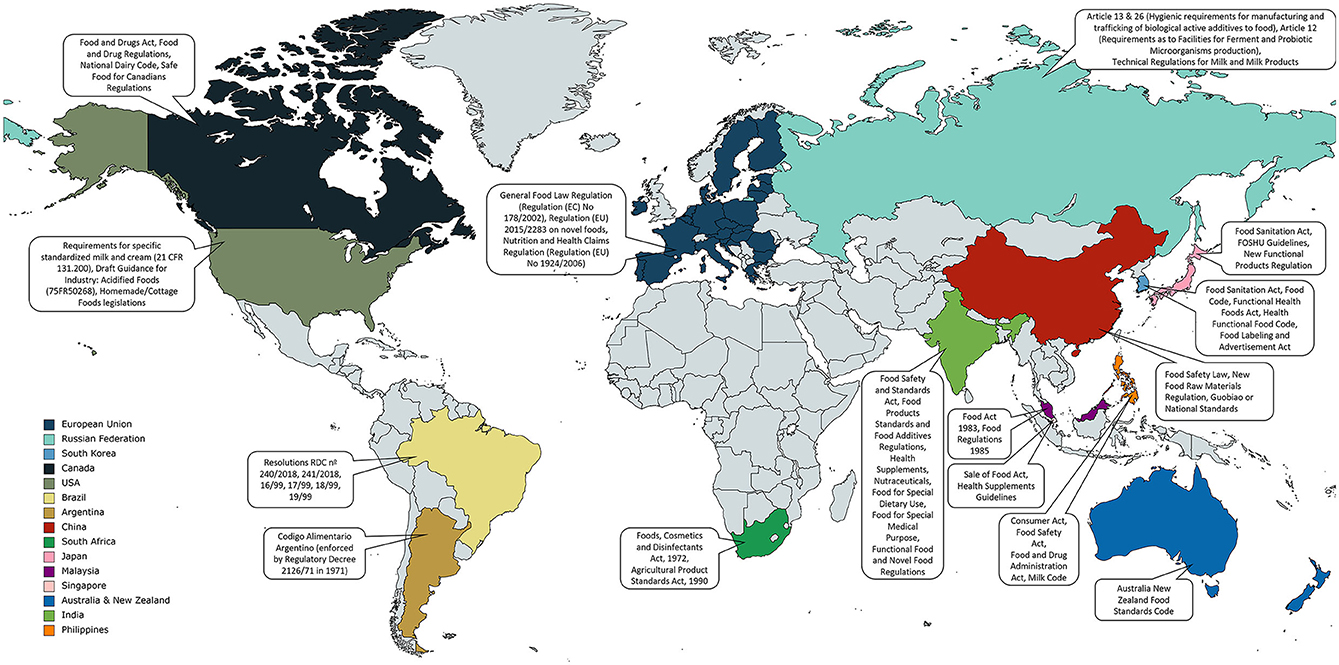
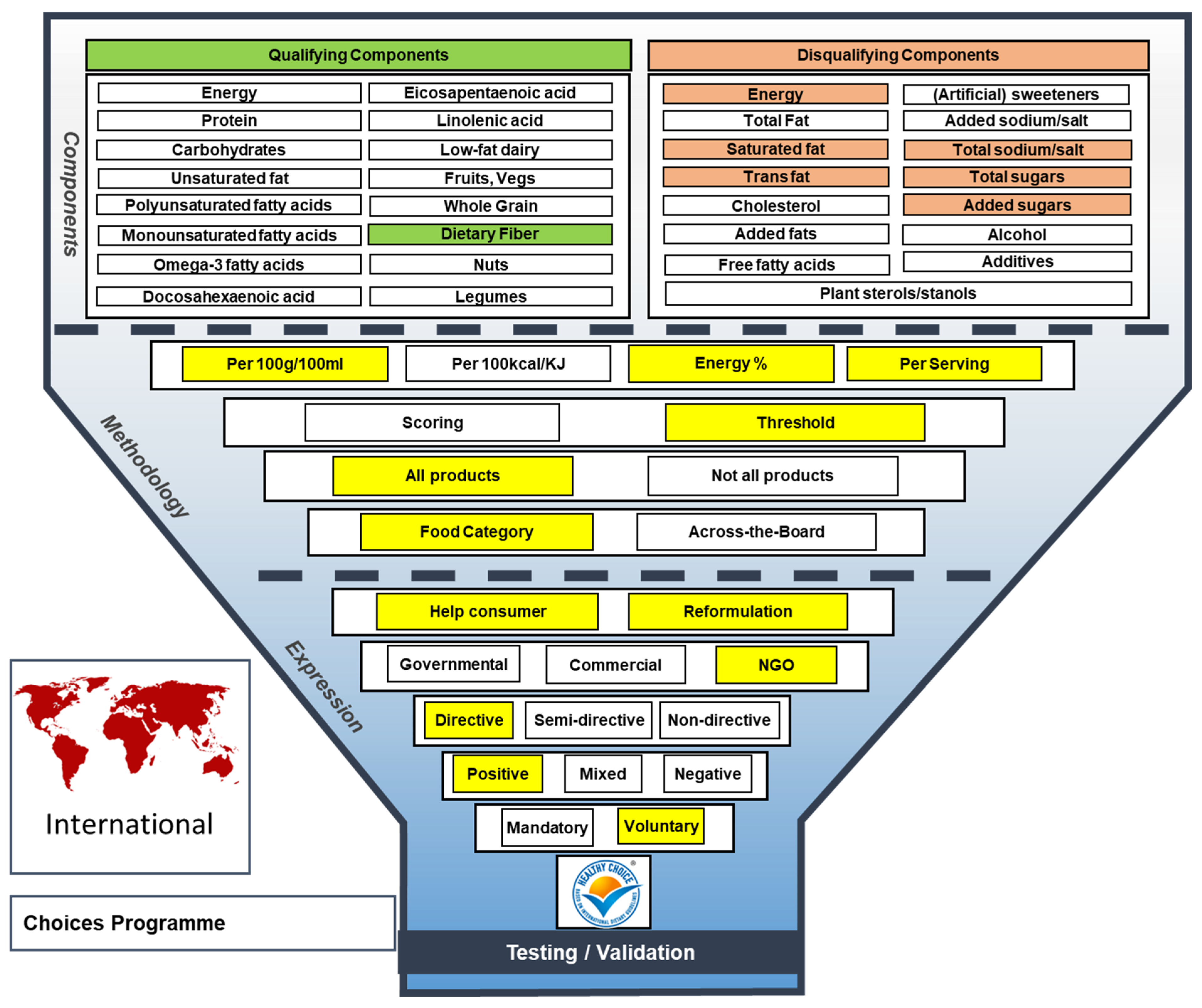
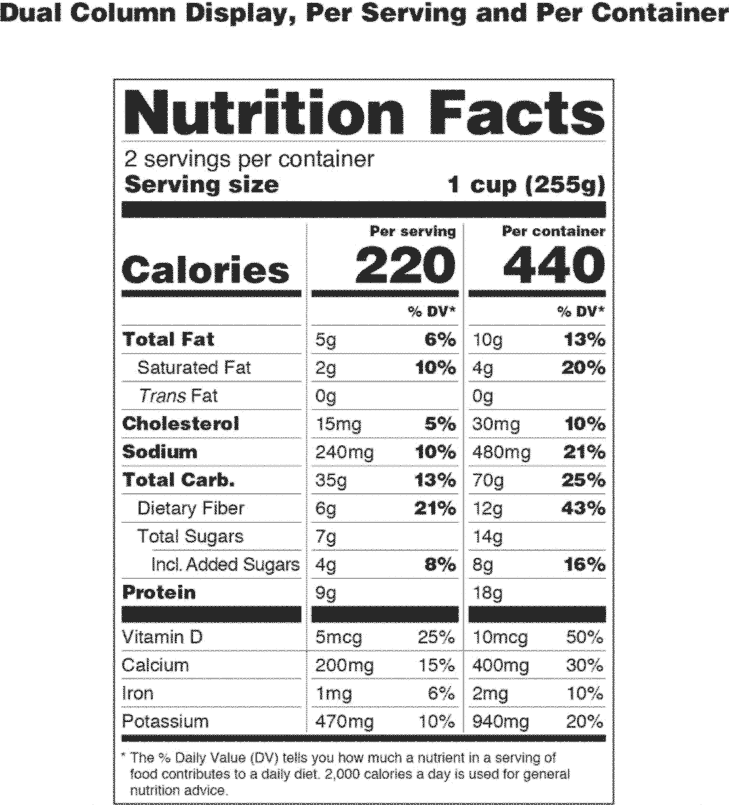



Post a Comment for "42 a product featuring a qualified health claim on its label:"