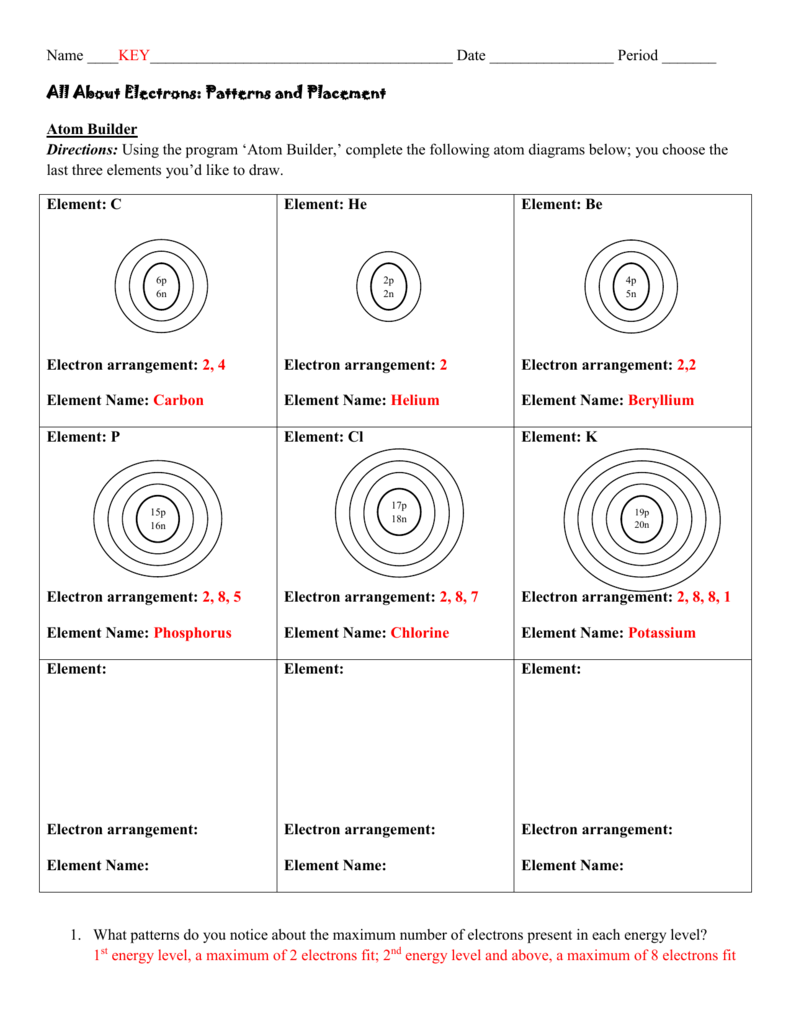
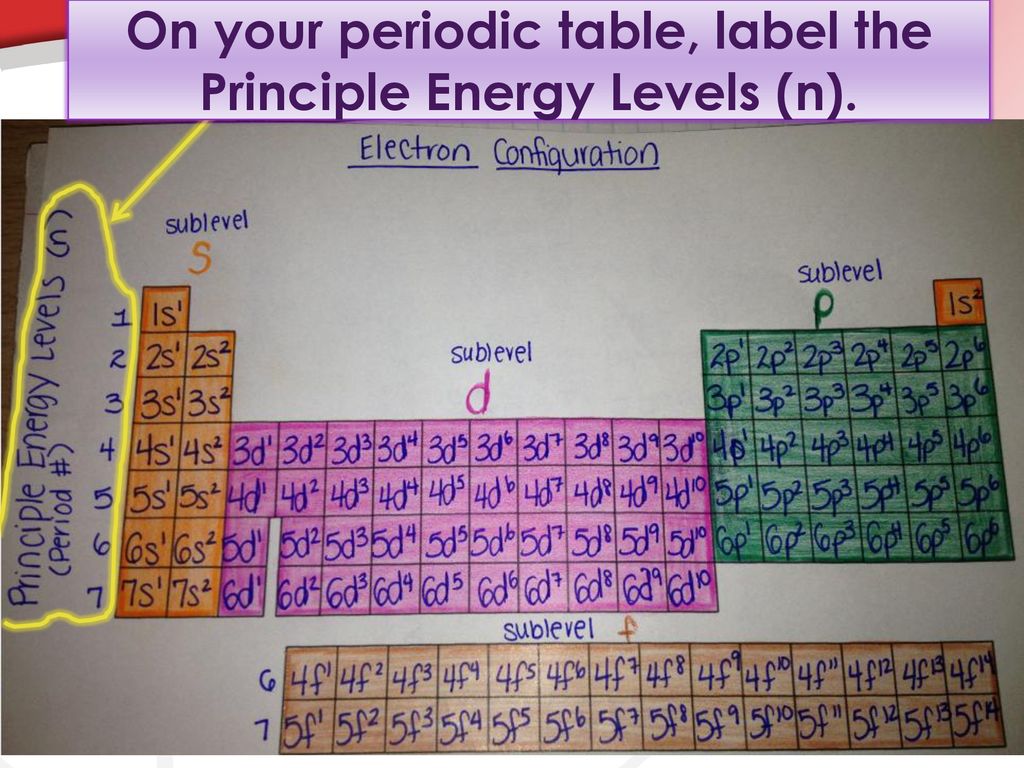
44 what is used to label the energy levels of electrons
Atomic Energy Levels (video) | Khan Academy The electron can absorb photons that will make it's charge positive, but it will no longer be bound the the atom, and won't be a part of it. For example at -10ev, it can absorb, 4eV (will move to -6eV), 6eV (will move to -4eV), 7eV (will move to -3eV), and anything above 7eV (will leave the atom) 2 comments ( 12 votes) Upvote Downvote Flag more 5.12: Energy Level - Chemistry LibreTexts Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Electrons are tiny, negatively charged particles in an atom that move around the positive nucleus at the center. Energy levels are a little like the steps of a staircase.
The Periodic Table and Energy-Level Models - Middle School Chemistry The electrons surrounding an atom are located in regions around the nucleus called "energy levels". An energy level represents the 3-dimensional space surrounding the nucleus where electrons are most likely to be. The first energy level is closest to the nucleus. The second energy level is a little farther away than the first.
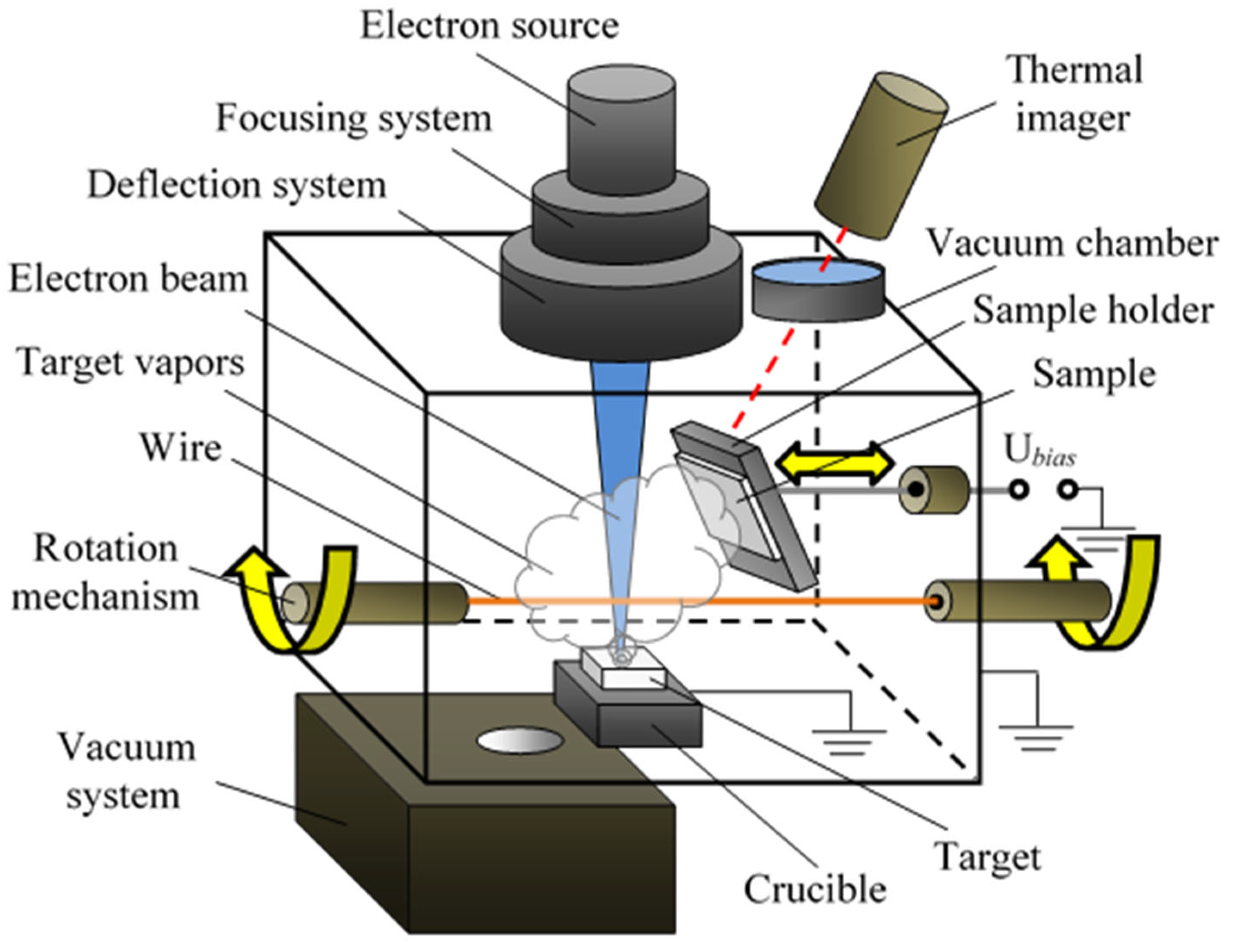
What is used to label the energy levels of electrons
The periodic table, electron shells, and orbitals - Khan Academy Most of the elements important in biology need eight electrons in their outermost shell in order to be stable, and this rule of thumb is known as the octet rule. Some atoms can be stable with an octet even though their valence shell is the 3n shell, which can hold up to 18 electrons. Photoelectron spectroscopy (article) | Khan Academy Summary. Photoelectron spectroscopy (PES) is an experimental technique used to determine the relative energies of electrons in atoms and molecules. Photoelectron spectrometers work by ionizing samples using high-energy radiation (such as UV or x-rays) and then measuring the kinetic energies (. KE. Bohr's model of hydrogen (article) | Khan Academy The Balmer series—the spectral lines in the visible region of hydrogen's emission spectrum—corresponds to electrons relaxing from n=3-6 energy levels to the n=2 energy level. Bohr could now precisely describe the processes of absorption and emission in terms of electronic structure.
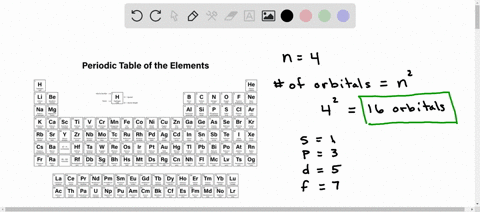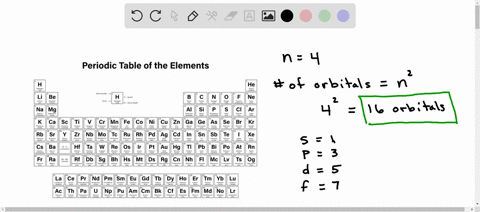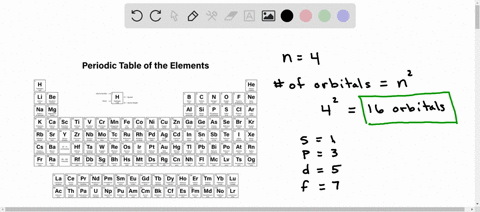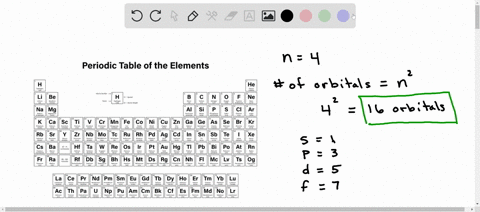
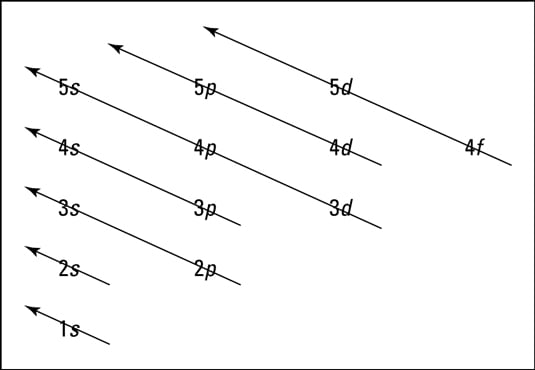
What is used to label the energy levels of electrons. How to Represent Electrons in an Energy Level Diagram An energy level diagram is more useful and easier to work with than quantum numbers in the quantum mechanical model. Chemists use the energy level diagram as well as electron configuration notation to represent which energy level, subshell, and orbital are occupied by electrons in any particular atom. Chemists use this information in these ways: Solved 7. What is the term used to label the energy levels - Chegg What is the term used to label the energy levels of electrons? 8. How are s orbitals different from p orbitals? 9. How many electrons can each of the following orbitals hold? a. 2 s=22 d. 6 d=10 b. 3p=146 c. 5f= 10. How many "p" orbitals can there be in any energy level? 11. What is the maximum number of electrons in the 3nd principle energy level? Chapter 5 Electrons in Atoms Flashcards | Quizlet What are the fixed energies of electrons called? energy levels Circle the letter of the term that completes the sentence correctly. A quantum of energy is the amount of energy required to a. place an electron in an energy level. b. maintain an electron in its present energy level. c. move an electron from its present energy level to a higher one. C What term is used to label the energy levels of electrons? Answer : The term used to label the energy levels of electrons is, Principle Quantum Numbers (n). Explanation : There are 4 quantum number. Principle Quantum Numbers : It describes the size of the orbital or energy levels of electrons. It is represented by n. n = 1,2,3,4.... Azimuthal Quantum Number : It describes the shape of the orbital.
Counting valence electrons for main group elements The octet rule follows from the energetic favorability of having both the s and p subshells either completely empty or completely full. There is no p subshell in shell number 1, so that is part of why the lightest elements do not follow the octet rule (there are other reasons). Lesson Explainer: Electrons and Energy Levels | Nagwa The maximum number of electrons changes dramatically from one energy level to the next. The first energy level can contain 2 electrons, and the third and fourth levels can hold up to 18 and 32 electrons. Mathematicians would describe the increase as exponential. Scientists do not ordinarily apply this equation to levels higher than the fourth one. Circle the letter of the term that is used to label the ener - Quizlet In the quantum mechanical model, electron energy levels are denoted by principal quantum numbers (nnn). These are given the values nnn= 1, 2, 3, 4, and so on. Answered 1 year ago Step 1 1 of 4 In general, the energyof a given electron is related to its distancefrom the nucleus. Energy level - Wikipedia A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels.This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can ...
Energy Level and Transition of Electrons - Brilliant The energy level of the electron of a hydrogen atom is given by the following formula, where n n denotes the principal quantum number: E_n=-\frac {1312} {n^2}\text { kJ/mol}. E n = − n21312 kJ/mol. For a single electron instead of per mole, the formula in eV (electron volts) is also widely used: E_n=-\frac {13.6} {n^2}\text { eV}. What is the term used to label the energy levels of electrons? The term used to label the energy levels of electrons is called the electron energy levels. The energy levels of electrons are important because they determine how electrons behave. The energy levels of electrons determine how electrons interact with other particles, how they absorb and emit energy, and how they move around. Bohr Diagrams of Atoms and Ions - Chemistry LibreTexts These energy levels are designated by a number and the symbol "n." For example, the 1n shell represents the first energy level located closest to the nucleus. Figure \(\PageIndex{1}\): The Bohr model postulated that electron orbited the nucleus in shells of fixed distance. An electron normally exists in the lowest energy shell available, which ... 3.3.4: Assembling a complete MO diagram - Chemistry LibreTexts Label the energy levels (sigma, pi, etc.) and add in the correct number of electrons. Show how to calculate the bond order in the molecule. Answer. 3.3.4: Assembling a complete MO diagram is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts.
Chapter 5 test Section 5.1 Flashcards | Quizlet The electrons in an atom can exist between energy levels. Energy levels What are the fixed energies of electrons called? Move an electron from its present energy level to a higher one A quantum of energy is the amount of energy required to? Closer In general, the higher the electron is on the energy ladder, the ______ it is from the nucleus
Bohr model energy levels (video) | Khan Academy So, we have the energies for three different energy levels. The energy for the first energy level is equal to negative 13.6. E two is equal to negative 3.4, and E three is equal to negative 1.51 electron volts. So energy is quantized using the Bohr models, you can't have a value of energy in between those energies.
Bohr's model of hydrogen (article) | Khan Academy The Balmer series—the spectral lines in the visible region of hydrogen's emission spectrum—corresponds to electrons relaxing from n=3-6 energy levels to the n=2 energy level. Bohr could now precisely describe the processes of absorption and emission in terms of electronic structure.
Photoelectron spectroscopy (article) | Khan Academy Summary. Photoelectron spectroscopy (PES) is an experimental technique used to determine the relative energies of electrons in atoms and molecules. Photoelectron spectrometers work by ionizing samples using high-energy radiation (such as UV or x-rays) and then measuring the kinetic energies (. KE.
The periodic table, electron shells, and orbitals - Khan Academy Most of the elements important in biology need eight electrons in their outermost shell in order to be stable, and this rule of thumb is known as the octet rule. Some atoms can be stable with an octet even though their valence shell is the 3n shell, which can hold up to 18 electrons.
























:max_bytes(150000):strip_icc()/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)

Post a Comment for "44 what is used to label the energy levels of electrons"